2Ap01:
AAVLP-HPV
2AP01 – Cervical, skin, oropharyngeal and other HPV-related cancers
A new HPV vaccine for a growing market
OVERVIEW:
- The world’s only L2 based HPV vaccine in clinical testing
- Significantly broader coverage than approved HPV vaccines
- First-in-human, clinical phase 1 trial completed
- Cost-effective as only one AAVLP particle type required for cross-neutralising protection
INFORMATION:
Human Papillomavirus (HPV) is a group of viruses that infects epithelial cells in the mucosa causing cervical and cutaneous cancers, among others. More than 170 types of HPV have been fully sequenced and there are many more to be investigated. The most advanced currently approved HPV vaccine provides protection against nine alpha-HPV types. However, there are still 300,000 preventable cervical cancer deaths per year alone. Furthermore, alpha-HPV types that are not covered by current vaccines cause about 10% of cervical, vaginal, anal, vulvar, penile, head and neck cancers, genital warts and recurrent respiratory papillomatosis. Beta-HPV types, on the other hand, have shown to be associated with the development of nonmelanoma skin cancers (NMSCs) including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) and are still unaddressed.
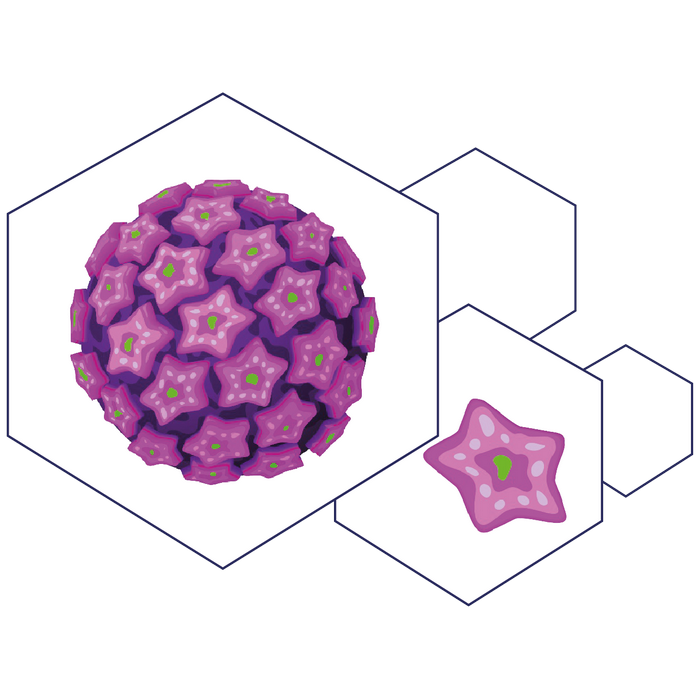
A demonstration of a Human Papillomavirus (HPV). The HPV capsid consists of 360 copies of the major capsid protein, L1, and arranged as pentamers (pink), with substoichiometric amounts of the minor capsid protein, L2 (green).
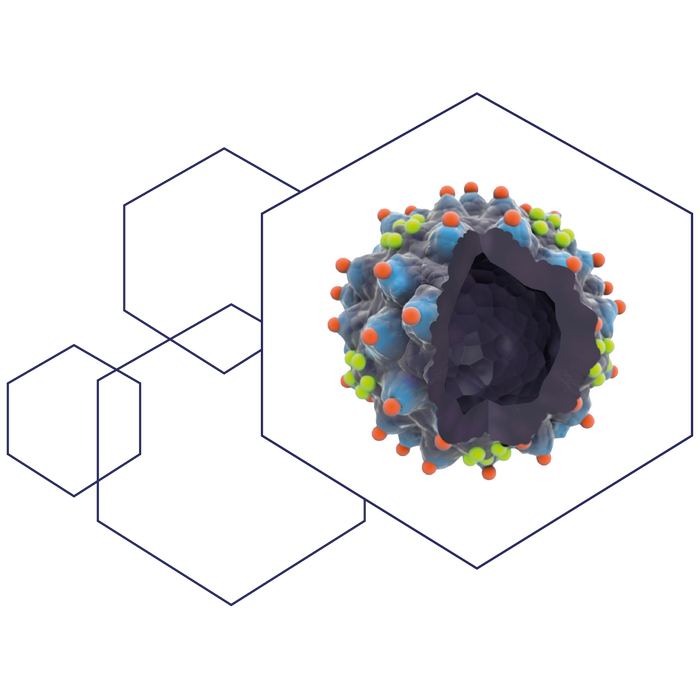
This illustration Demonstrates the AAVLP-HPV vaccine displaying the HPV3 1 L2 epitope in position 453 (red spheres) and the HPV16 L2 epitope in position 587 (green spheres).
The global market for prophylactic HPV vaccines is expected to grow rapidly to more than USD $5.6 billion by 2023(2) as more countries expand national vaccination programs to include HPV vaccinations, not only for girls but also for boys. In addition, it is now estimated that 60-70% of oropharyngeal cancers are caused by HPV virus infections, opening a discussion on HPV vaccinations for adults as well. NMSC development is highly prevalent in solid organ transplantation recipients (40% in Northern Europe and 70% in Australia will develop NMSC after 20 years3,4). Up to 100% of transplanted patients have subsequent NMSCs in the 5 years after the primary NMSC, leaving a huge market unaddressed.
2A Pharma is in collaboration with Johns Hopkins University to bring this vaccine to market.

Relevant publications:
1: Harwood, C.A., 2017.
2: Datamonitor & WHO Human Papillomavirus Vaccine Supply and Demand Update, October 2020
3: Bouwes Bavinck JN, Claas FH, Hardie DR, Green A, et al. Relation between HLA antigens and skin cancer in renal transplant recipients in Queensland, Australia. J Invest Dermatol 1997;108:708–11.
4: Bouwes Bavinck JN, Hardie DR, Green A, Cutmore S, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia A follow-up study. Transplantation
1996;61:715–21.
