SCIENTIFIC
FOUNDATION
2A Pharma’s patented AAVLP platform technology
2A Pharma’s vaccine platform is developed from adeno-associated virus-like particles, also called AAVLPs. Vaccines based on the virus-like particle (VLP) technology have been used for gene therapy applications for decades but have first entered the vaccine technology field during the last two decades. Currently, a handful of prophylactic VLP based vaccines have been commercialised worldwide providing safer and cheaper vaccines than earlier.
VLPs are non-infectious scaffolds of proteins mimicking the virus repetitive structure. Introducing these into the body will trigger both a cellular and humoral immune response resulting in the generation of antibodies and thereby immunogenic protection. Importantly, VLPs do not contain any viral genomic material and are unable to replicate therefore being non-infectious and very safe.
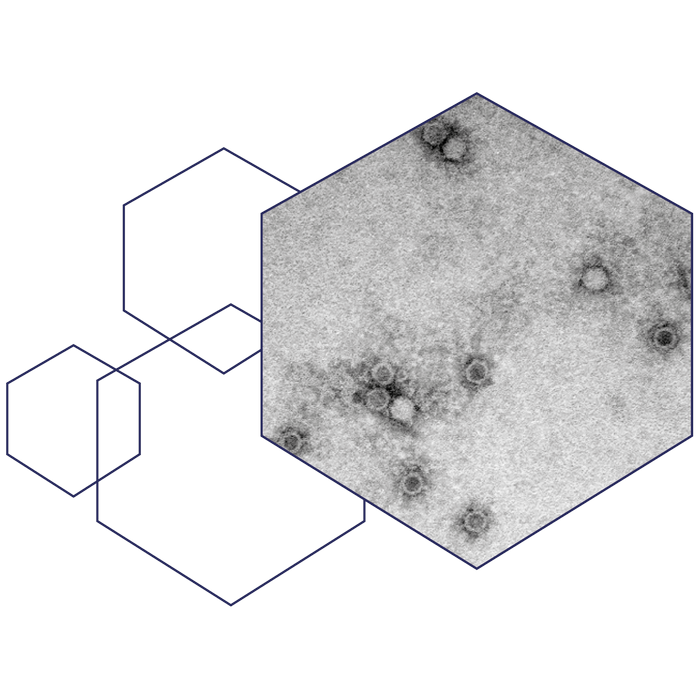
A transmission electron microscopy image of the AAVLP vaccine
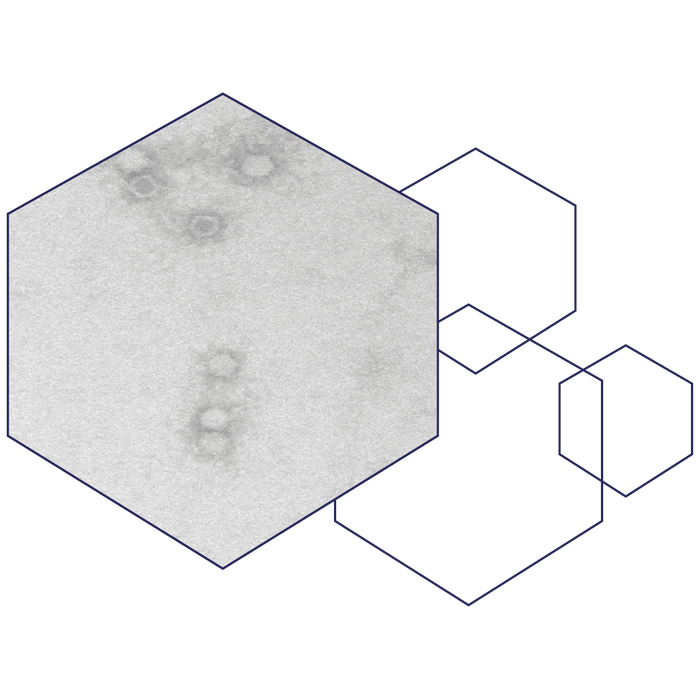
The virus-like particle used in our AAVLP vaccine platform is derived from an adeno-associated virus type 2 (AAV2), hence the name. AAV2 are small, ssDNA, non-enveloped viruses capable of infecting a range of vertebrates including humans and other primates. AAVs belong to the Parvoviridae family in the Dependovirus genus. They require co-infection with adeno- or herpesviruses to be able to replicate and are not known to cause disease.
A wildtype AAV consists of a 4.7 kb genome encoding non-structural (rep), structural (cap), assembly activating (aap), and membrane associated accessory (maap) proteins. The AAV capsid are composed of a total of 60 molecules of the structural viral proteins (VPs) in a ratio of 1:1:8 of VP1, VP2, and VP3, respectively. 2A Pharma has modified the wildtype AAV2 into AAVLPs that only consist of VP3 capsid proteins and assembly activating proteins to form empty, structural shells without any viral genomic material. Each VP3 sequence encompasses two dominant immunogenic sites, at position 453 and 587, where epitopes up to 34 amino acids of interest can be integrated as a part of the capsid structure.
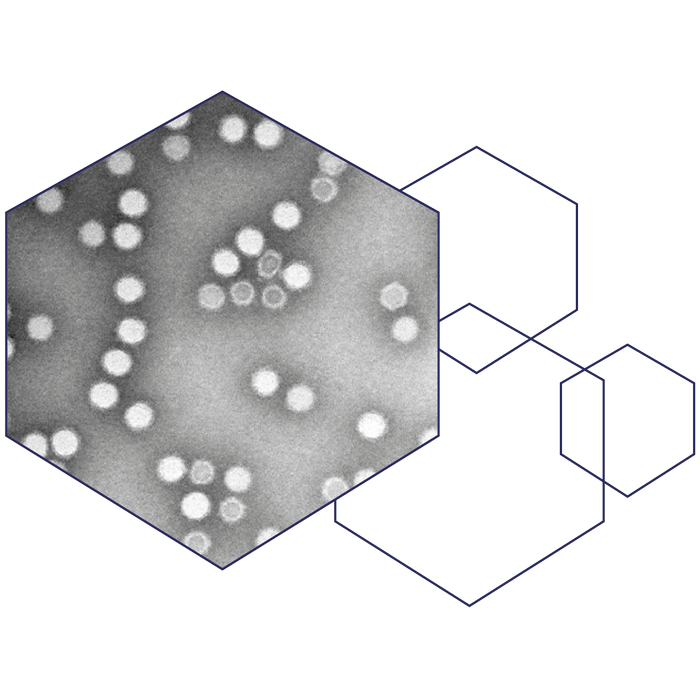
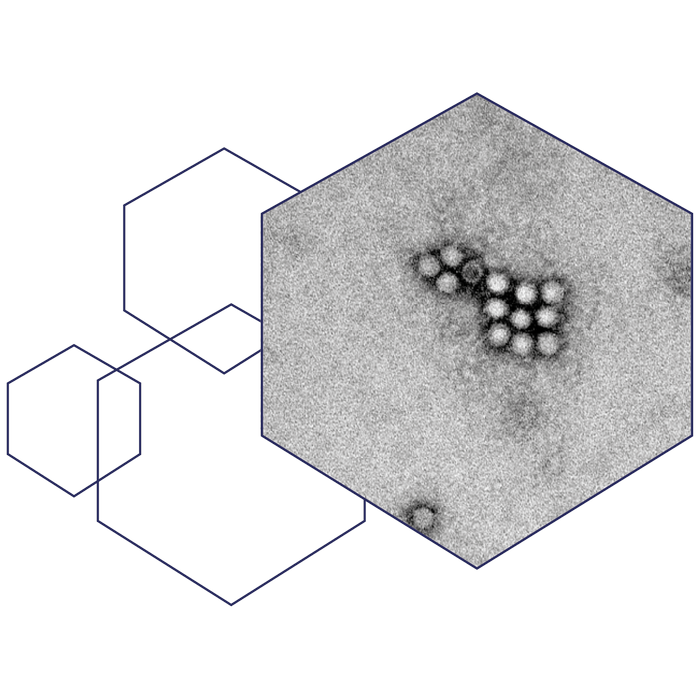
The two immunogenic positions make it possible to develop both monovalent and bivalent vaccines as the two positions can be used together in one vaccine. In theory, this integration of epitopes into the capsid structure, makes it possible for us to direct the immune response to a plethora of diseases/pathogens as the epitope(s) can be changed to target any disease of interest. An important advantage of the AAVLP platform is the capability of turning non-immunogenic peptides into immunogenic antigens. This enables us to incorporate two conserved epitopes that are, phylogenetically, far from each other, to reach a broad, cross-protective immune response and development of pan-protecting vaccines against a whole family of viruses regardless of mutations.
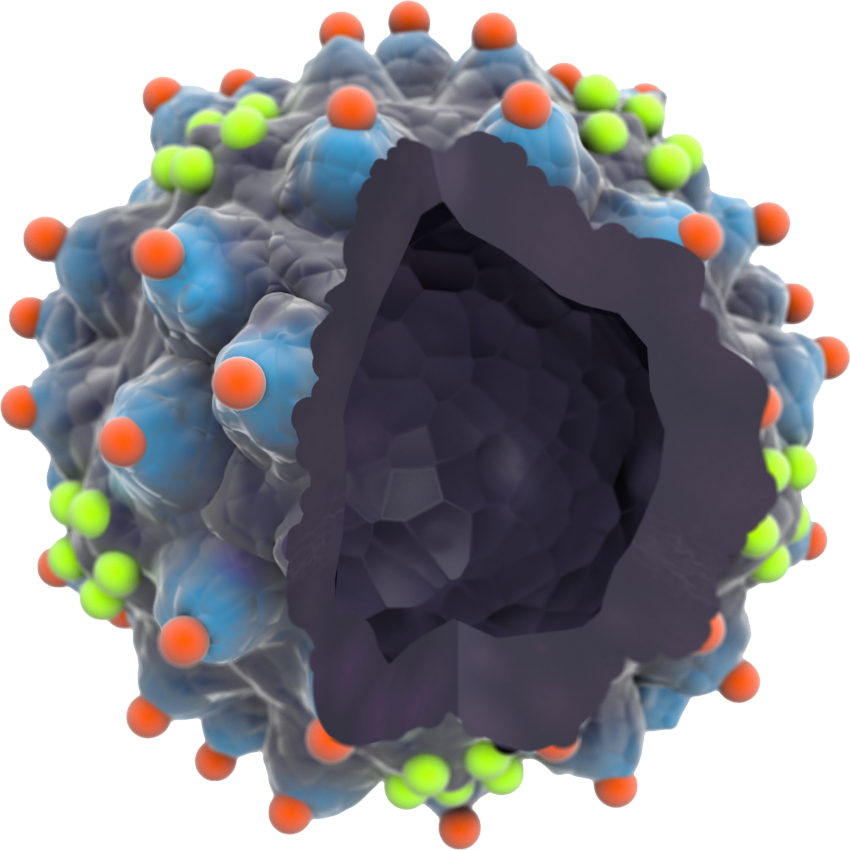
An AAVLP with Epitope cloning
No viral genome inside the AAVLP.
Peptides inserted in relevant peaks and valleys shown in red and green to stimulate the right immune response.
Utilising the AAVLP platform as a vaccine carrier holds unique properties:
- The AAVLP capsid forces the epitopes into a fixed three-dimensional structure with a distance optimal for efficient B-cell receptor recognition. Recognising the epitope in a conformational structure generates a protein-specific immune response rather than a peptide-specific response being more robust and broader.
- The structural arrangement together with the multivalent display of epitopes enhances cross-linking of B-cell receptors on naïve B-cells, leading to a direct and strong B-cell activation, proliferation, and differentiation, secretion of high-affinity antibodies, and the generation of long-lasting plasma cell activation and memory induction.
- Already, fourteen days after prime vaccination immunoglobulin (Ig)G are detectable in human serum.
- Due to the inherited immunogenicity of AAV, low immunogenic epitopes are turned into highly immunogenic antigens. This property is of high importance when targeting conserved domains, as these are often hidden and low immunogenic, compared to exposed proteins which are normally used as target in vaccine technology.
- AAVLPs are between 25 to 30 nm in diameter. This size is favourable for being able to enter the lymphatic system directly through diffusion of cell junctions for efficient exposure to immune cells. AAVLPs are also taken up and transported by antigen-precenting cells (APCs) for presentation to T-helper cells.
- 80-90% of the human population is seropositive for several AAV serotypes, crucially, this pre-existing Th cell immune response boost the specific antibody response against the inserted epitopes
- The AAVLP platform is a protein vaccine and not a virus, therefore, it has a favourable safety profile.
- Two different antigenic epitopes can be integrated into the AAVLP capsid, generating a bivalent AAVLP-vaccine, enabling a broader protection.
- AAVLPs are fast and cost-effective to produce.
Relevant Publications
1) Carlander, AF et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: A Danish population-based study from 2011 to 2014. European Journal of Cancer, 70:75-82, January 2017
2) Chouhy D, Bolatti E.M., Perez G.R., Giri A.A. Analysis of the genetic diversity and phylogenetic relationships of putative human papillomavirus types Journal of General Virology 94: 2480–2488, November 2013
3) DelveInsight. Human Papillomavirs (HPV) Prophylaxis Landscape. Research Report, 2017
4) Gambhira, R. et al. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. Journal of Virology, vol. 81(24), December 2007
5) Jagu, S., Pino Tossi, K., Roden, R.B.S., Christensen, N.D. et al. Durable immunity to oncogenic human papillomaviruses elicited by adjuvanted recombinant Adeno-associated virus-like particle immunogen displaying L2 17–36 epitopes HHS Public Access. Vaccine, 33(42): 5553–5563, 13 October 2015
6) Kronenberg, S. et al. Electron cryo-microscopy and image reconstruction of adeno-associated virus type 2 empty capsids. German Cancer Research Center, Heidelberg, Germany, 2 November 2001
7) Manzano-Szalai, K. et al. Adeno-Associated Virus-Like Particles as New Carriers for B-Cell Vaccines: Testing Immunogenicity and Safety in BALB/c Mice. Viral Immunology, Volume 27, Number 9, pp. 438-448, 2014
8) Nieto, K., Hörer, M., Roden, R.B.S. et al. Development of AAVLP(HPV16/31L2) Particles as Broadly Protective HPV Vaccine Candidate. German Cancer Research Center, Heidelberg, Germany, 27 June 2012
9) Schellenbachera, C., Roden, R.B.S., Kirnbauera, R. Developments in L2-based human papillomavirus (HPV) vaccines. Virus Research, 23 November 2016
10) Denman CJ, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 128(8):2041-8, 2008
11) Jacquemin C, et al. HSP70 potentiates interferon alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. Br J Dermatol. doi: 10.1111/bjd.15550, 2017
12) Malyshev I. Immunity, Tumors and Aging: The Role of HSP70. Dordrecht, Heidelberg, 20 New York, London: Springer. 63-82, 2013
13) Mosenson JA, e. al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 5(174):174ra28, 2013
14) Mosenson JA, et al. Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress. Pigment Cell Melanoma Res. 27(2):209-20, 2014
15) Wang D, et al. Human keratinocytes release high levels of inducible heat shock protein 70 that enhances peptide uptake. Exp Dermatol. 20(8):637-41, 2011
